Umxholo
Inkqubo ye- ezisisiseko oxides, kwaziwa njenge ii-oxide zentsimbi, zezi zidibanisa ioksijini kunye nezinto zentsimbi. Kuba ioksijini ine-elekhtronegative kwaye isinyithi sikhetha ubuchwephesha, ibhondi esekwe yi-ionic.
Inkqubo ye- ifomula elemental emele zonke i-oxides ezisisiseko yi-XO, apho i-X sisixhobo se-metallic kunye ne-O oksijini. Nganye kwezi inokulandelwa yimirhumo (ngokubanzi eyi-2 okanye ye-3), ebonakala ngokutshintsha i-valence (Oko kukuthi, leyo yesinyithi kunye neoksijini).
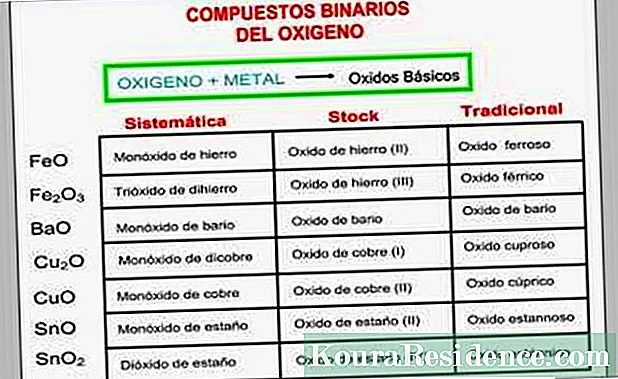
Uluhlu lwamagama ezinto ezisisiseko ze-oksidi
Isidliso sendabukoIi-oxides ezisisiseko zithiywa ngokukhankanya okokuqala igama elithi "oxide ye" kunye negama lento yesinyithi, okanye "i-oxide" elandelwa sisichazi eligama lelungu lesinyithi elinokupheliswa okwahlukileyo, njengoko kucacisiwe apha ngezantsi:
- Kwiizinyithi ezinodidi olunye kuphela lwe-valence (njenge-sodium okanye i-calcium), icandelo lesinyithi lakhiwa njengegama esdrújula elinesiphelo "ico".
- Kwizinyithi ezikhoyo iintlobo ezimbini ze-valence . Ukuba ibandakanya eyona valence iphezulu, igama lesinyithi longezwa kunye nesimamva "ico" kwaye ligama elithi esdrújula.
- Xa ikhona valence ezintathu ezinokwenzeka (njenge chromium), ukuba i-oxide ibandakanya i-valence esezantsi, igama lesinyithi longezwa kwisimaphambili "hiccup" kunye nesimamva "ibhere", kwaye ligama elibi. Xa ibandakanya i-valence ephakathi, intsimbi ibizwa ngegama "ibhere" ephelayo kwaye iseligama elibi, kodwa ukuba ibandakanya eyona valence iphezulu, isiphelo ngu "ico" kwaye ligama le-sdrújula.
- Isinyithi inayo zine ezinokubakho (njenge manganese), iskim siyafana naleya yangaphambili kwezintathu zokuqala, kodwa xa isinyithi sidityaniswe kwi-oxide kunye ne-valence yesine kunye neyona ephezulu, igama lesinyithi longezwa nesimaphambili "nganye" kunye Isimamva "ico", kwaye ligama elithi esdrújula.
Isidweliso segamaIsitokhweNgaphantsi kwesi sigama, ii-oxide zibhaliwe kwaye zibizwa ngokuba yi "oxide of" + element metallic + Roman numeral in parentheses, ebonisa ubumdaka apho isinyithi isinxibelelana neoksijini.
Inkqubo yokuchazwa ngegamaOkwangoku kukhethwa yi IUPAC(UManyano lwaMazwe ngaMazwe lweKhemistri eQhelekileyo nesetyenzisiweyo), ingqikelelo yokubabiza ngokuba zi "oxides ze" iyagcinwa, kodwa ukwenza njalo ngokuchanekileyo ngokudibanisa isimaphambili esiqhelekileyo sesiGrike esihambelana nenani leeathom zeoksijini (kwigama elithi "oxide") kunye nenani leeathom zesinyithi (kwi igama lesinyithi) equlethwe yimolekyuli nganye, kusetyenziswa isimaphambili "sika" njengebhulorho.
I-oxides ezisisiseko zisetyenziselwa ukubala, ipeyinti, izinto zokwakha, iiplastiki kunye namanye amashishini.
Imizekelo yee-oxides ezisisiseko
| idialuminium trioxide | manganous oxide |
| icobalt oxide | i-permanganic oxide |
| ikomityi oxide | calcium oxide |
| oxide hypochromic | zinc oxide |
| intsimbi eyintsimbi | chrome oxide |
| i-ferric oxide | chromic oxide |
| i-magnesium oxide | oksidi yemekyuri |
| umhlwa | i-dimanganese trioxide |
| oxide eyomeleleyo | I-dicobalt trioxide |
| I-stannic oxide | titanium dioxide |
Ezinye iintlobo ze-oxides:
- Izinyithi zentsimbi
- IiOxides ezingezizo ezentsimbi
- Iiasidi zeOididi


